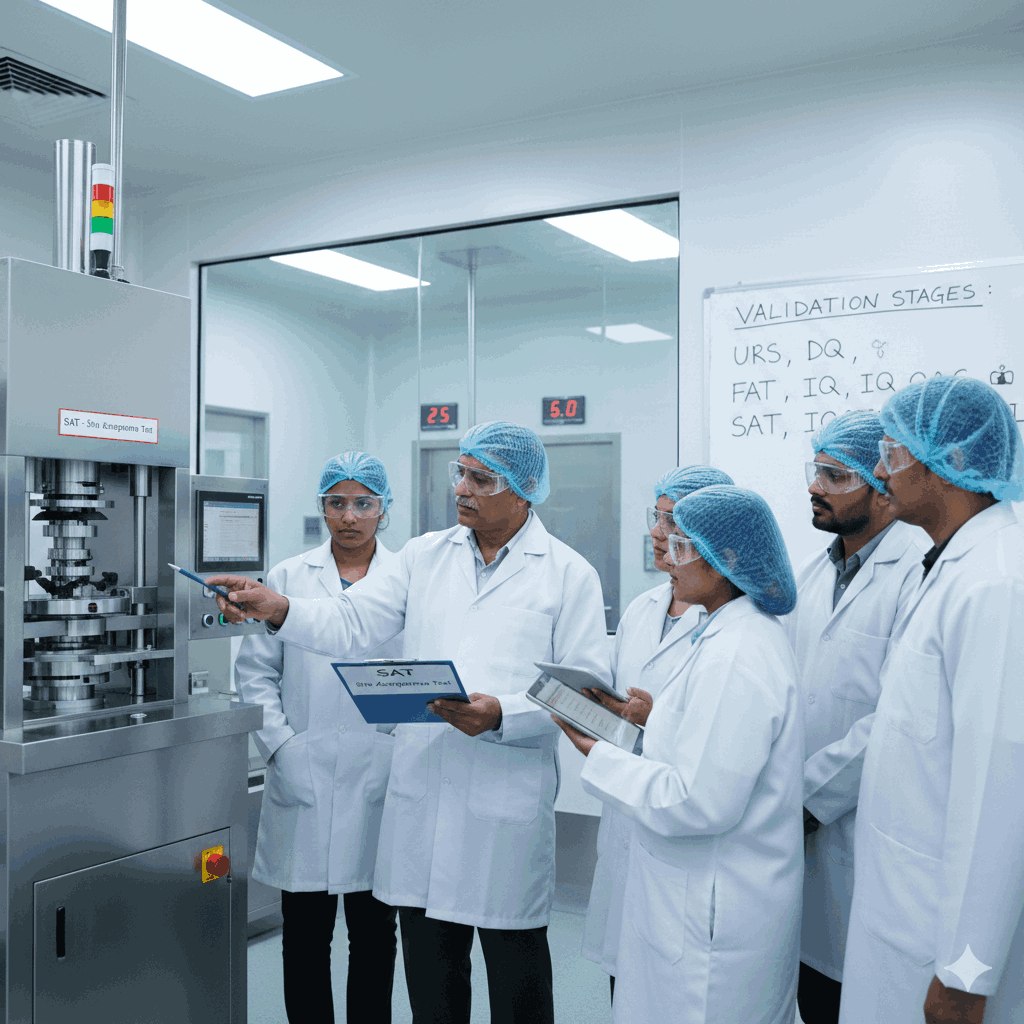
Execution & Documentation as per GMP Standards
All project activities are carried out and documented as per GMP standards to maintain quality and follow the required rules. Each stage is clearly defined and recorded, so the process remains easy to track and manage.
-
Documents Include:
- User Requirement Specifications (URS)
- Functional Specifications (FS)
- Design Qualification (DQ)
- Factory Acceptance Test (FAT)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Satisfactory Acceptance Test (SAT)
Site Master File (SMF)
The Site Master File (SMF) gives a clear overview of the manufacturing facility. It covers infrastructure, quality management systems, and daily operations.
This document shows how the site follows regulatory requirements to maintain product safety, quality, and consistency. Regulatory authorities use the SMF as a reference during inspections and audits.

Benefits of Execution & Documentation for Food Industry
Better Compliance

Clear Records

Smooth Audits
Quality Assurance
Contact Us Today!
Ready to get the right execution and documentation support for your project?
FAQ's
Frequently Asked Questions
What is Execution & Documentation in the food industry?
Ans. It is the process of carrying out project tasks and recording them properly to maintain quality and follow GMP standards.
Why is documentation important for food projects?
Ans. Documentation provides a clear record of processes, equipment, and tests, helping maintain product quality and safety.
What documents are included in Execution & Documentation?
Ans. Key documents include URS, Functional Specifications, Design Qualification, FAT, IQ, OQ, and SAT.
How does Execution & Documentation help during audits?
Ans. It gives auditors complete and organised records, making the audit process easier.